We produce complete medical technology appliances, which are ready for sale, labelled, packaged and documented. |



|
We produce electronic and electromechanical medical technology modules, which are ready to install, tested and documented in accordance with EN ISO 13485 and ISO 9001 in small to medium quantities. |



|
| By using modern aids in construction and product design, we are able – despite the increasing complexity of our products – to reduce the time to market considerably and react to diminishing market cycles. |



|
| We also work with modern development tools in designing circuits and layouts. |




|
| Prototypes are produced in our own workshops as design and inspection samples, as are functional prototypes for tests and licensing purposes. |




|
| We assume responsibility for procuring components and managing them. This ensures that the right components are available at the right time and all processed parts are fully traceable. |

|
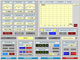
| Tests and inspections are required to meet our exacting quality requirements and the stringent safety requirements. We use calibrated measuring and testing equipment to carry them out. |


|
| We develop software in accordance with international standard IEC 62304 for medical technology products. |

|

